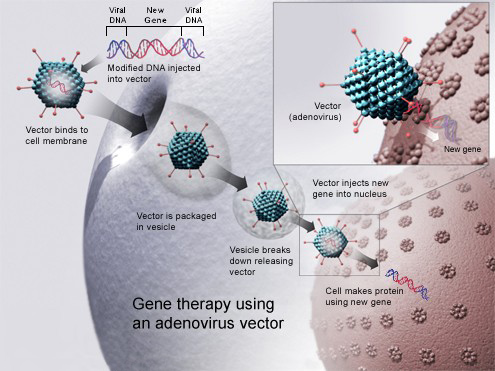
EPS is a Screening Site for new Gene Therapy LUXTURNA™
American Society of Retina Specialists (ASRS) reports that LUXTURNA™, a one-time gene therapy treatment, has been approved by the FDA. This is exciting news to retina specialists as they have new hope to offer those with IRD –more specifically a particular Inherited Retinal Disease. In order to receive this treatment a patient must be tested to see if they have viable retinal cells. We are happy to report that EPS is a screening site and we are well supplied with test kits. It is important to note that most of those who have confirmed gene mutation-associated retinal dystrophy will progress to blindness, therefore our Retina Specialists, Dr. Paula Ko and Dr. Carolyn Glazer-Hockstein, are excited to have this new treatment available. If you need to be tested for this therapy simply call 302-652-3353 and our reception group will be happy to get you scheduled.
ASRS offers the following sources of information about this treatment:
-
Foundation Fighting Blindness: http://www.blindness.org/
-
Info on gene therapy, genetic testing, living with IRDs: www.ASharedVision.com
-
Information on LUXTURNA™, ocular gene therapy treatment centers, and genetic testing program: www.Luxturna.com
-
Information on Spark patient support services: 1-833-SPARK-PS (1-833-772-7577).